full electron configuration for uranium|Complete Electron Configuration for Uranium (U) : Pilipinas Uranium sits amongst the actinides, the second shell of metals to fill their f-orbitals with valence electrons, making them large and weighty. Chemically, uranium is fascinating. . Fandom Apps Take your favorite fandoms with you and never miss a beat.
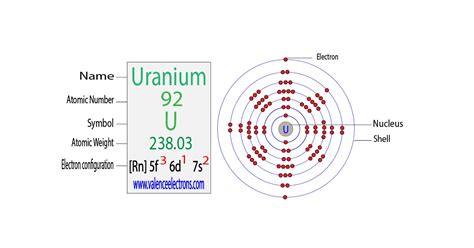
full electron configuration for uranium,The arrangement of electrons in uranium in specific rules in different orbits and orbitals is called the electron configuration of uranium. The electron configuration of uranium is [ Rn ] 5f 3 6d 1 7s 2 .Uranium is a chemical element of the periodic table with chemical symbol U and atomic number 92 with an atomic weight of 238.029 u and is classed as a actinide. The electron configuration for Uranium (U) is based upon the placement of uranium in the fourth column of the actinide series or f block. Mar 23, 2023 Uranium sits amongst the actinides, the second shell of metals to fill their f-orbitals with valence electrons, making them large and weighty. Chemically, uranium is fascinating. .The Electron configuration of uranium is [Rn] 5f3 6d1 7s2. Uranium is known as a chemical element that belongs to the periodic table of elements. Its atomic number is 92, it .
Uranium is the 92nd element in the periodic table and has a symbol of U and atomic number of 92. It has an atomic weight of (238.02891) and a mass number of 238. . From the orbital diagram, we can write the electron configuration in an abbreviated form in which the occupied orbitals are identified by their principal quantum .
Complete Electron Configuration for Uranium (U)Uranium metal can be prepared by reducing uranium halides with alkali or alkaline earth metals or by reducing uranium oxides by calcium, aluminum, or carbon at high .

The electron configuration of uranium is [Rn] 5f3 6d1 7s2. Electron configuration provides information about the distribution of electrons and their energy levels in an atom. . When we put in the full electronic configuration of the argon core, we find that the extra protons in the ferrous ion have pushed all the electrons into shell-by-shell order. Similarly, with $88$ . Uranium is a naturally radioactive element of the actinoid series, placed in the f-block element. Let us explore its electron configuration of it. The electron configuration of uranium is [Rn] 5f3 6d1 7s2. Its shell structure consists of 2,8,18,32,21,9,2. The chemical symbol of the uranium atom is “U,” having atomic .

But, the orbitals overlap. The Madelung rule gives the order: 1s < 2s < 2p < 3s < 3p < 4s < 3d < 4p < 5s < 4d < 5p < 6s < 4f < 5d < 6p < 7s < 5f < 6d < 7p. Oganesson (element 118) is a good example .Our expert help has broken down your problem into an easy-to-learn solution you can count on. Question: 33. (II) What is the full electron configuration for (a) nickel (Ni), (b) silver (Ag), (c) uranium (UD? (Hint: See the Periodic Table inside the back cover.) There are 2 steps to solve this one.full electron configuration for uranium Complete Electron Configuration for Uranium (U)According to the Aufbau principle, electrons fill the lowest energy orbitals first before moving to higher energy levels. Each subshell can accommodate a maximum number of electrons, which is determined by the formula 2n^2, where n represents the principal quantum number. In the case of uranium, the electron configuration is [Rn] 5f3 6d1 7s2.The electron configuration of an atom is the representation of the arrangement of electrons distributed among the orbital shells and subshells. . Uranium: Z:92 [Rn] 7s 2 5f 3 6d 1. Gold: Z:79 [Xe . We see that iodine has 5 electrons in the p orbitals. We know that the full p orbitals will add up to 6. Using the Hund's rule and Pauli .The electronic configuration of Uranium is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10 6s2 6p6 5f3 6d1 7s2. What is the abbreviated electronic configuration of Uranium? The abbreviated electronic configuration of Uranium is [Rn] 5f3 6d1 7s2. To form abbreviated notation of electronic configuration, the completely filled subshells . Uranium belongs to the actinide series and is metal in the silver-grey form. Uranium has both the 92 protons and the 92 electrons, out of which 6 are the valence electrons, and the rest 86 are the forms of other electrons. Uranium is having a very weak radioactive aspect as the isotopes of the Uranium are quite unstable. Period Table.Step 1. The full electron configuration for nickel (Ni) is 1 s 2, 2 s 2, 2 p 6, 3 s 2, 3 p 6, 4 s 2, 3 d 8. View the full answer Answer. Unlock. The uranium orbital diagram is a graphical representation of the electron configuration of the uranium atom. This diagram shows how the electrons in the uranium atom are arranged in different orbitals. Orbital is the region of space around the nucleus of an atom where electrons are found. To create an orbital diagram of uranium, you first .Part C What is the full electron configuration for uranium (U)? Express your answer in complete form as a string without blank space between orbitals. For example, 1s22s should be entered as 1s 22s"2. Submit Previous Answers Reguest Answer X Incorrect; Try Again; 4 attempts remaining . The electron configuration of lead is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 4f14 5s2 5p6 5d10 6s2 6p2, if the electron arrangement is through orbitals. . Therefore, the lead full electron .
Give the complete electron configuration for a uranium atom (careful scrutiny across the Periodic Table on the inside back cover will provide useful hints). physics How many different states are possible for an electron whose .
Electron atomic and molecular orbitals A Bohr diagram of lithium. In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. For example, the electron configuration of the neon atom is 1s 2 2s 2 2p 6, meaning that the 1s, 2s, and .
Inner transition elements are metallic elements in which the last electron added occupies an f orbital. They are shown in green in Figure 5.1.6 5.1. 6. The valence shells of the inner transition elements consist of the ( n – 2) f, the ( n – 1) d, and the ns subshells. There are two inner transition series: Electron Configuration and Oxidation States of Uranium. Electron configuration of Uranium is [Rn] 5f3 6d1 7s2. Possible oxidation states are +3,4,5,6. Electron Configuration. The periodic table is a tabular display of the chemical elements organized on the basis of their atomic numbers, electron configurations, and chemical .Now, we must define the uranium ground state electron configuration. Uranium, U is element of actinides, group of elements with unfilled f f f orbitals. We will start writing its electron configuration from the top. Remember that s s s subshell has 1 orbital, p p p subshell has 3 orbitals, while d d d subshell has 5 orbitals. The electron .Uranium Periodic table Plutonium . Neptunium in the periodic table. Symbol: Np: Atomic number . Electron configuration for neptunium. Electron configuration . Full configuration: 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 4f 14 5s 2 5p 6 5d 10 5f 4 6s 2 6p 6 6d 1 7s 2: Electron configuration chart. 1s 2: 2s 2: 2p 6: 3s 2: 3p 6: 3d 10 .
full electron configuration for uranium|Complete Electron Configuration for Uranium (U)
PH0 · electron configuration of uranium
PH1 · What is uranium's electron configuration? Chemistry Q&A
PH2 · What is uranium's electron configuration?
PH3 · Uranium (U)
PH4 · Uranium
PH5 · Periodic Table of Elements: Los Alamos National
PH6 · How To Write Electron Configuration For Uranium
PH7 · Electron Configuration Chart of All Elements (Full Chart)
PH8 · Complete Electron Configuration for Uranium (U)
PH9 · 7.8: Electron Configurations